
Polyurethanes are a wide and versatile range of coating in the paint industry, the unique features of polyurethane have made it attract the attention of many consumers, and polyurethane paints have several features that we can resist. We should point out that them are resistant to moisture, chemicals, and stickiness and scratch resistance. If we want to define polyurethane, we say that what is considered in polyurethane is related to the type of intermolecular reaction of its components that produces covalent bonds. And the urethane functional group is formed by a mechanism in organic chemistry.The urethane bond always takes place between the hydroxyl-OH functional group and the isocyanate-NCO functional group in the presence of a Lewis acid catalyst or type 3 amines.
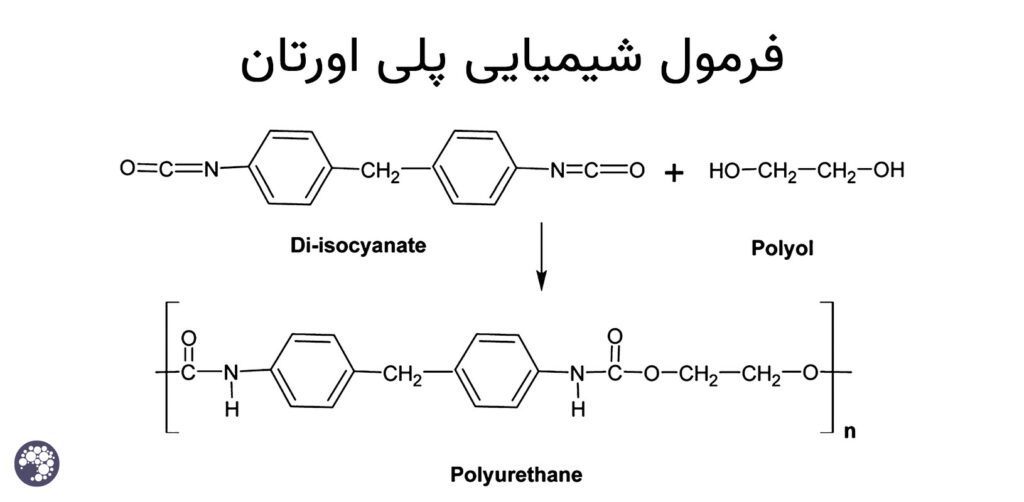
As seen in the urethane formation reaction, this reaction does not release any byproducts such as water, methanol and carbon dioxide caused by the reactions, and in polymer science, this is a very special advantage in the formation of a polymer film because this property of polyurethane frees it from depending on the environment conditions to a great extent, for example, the reaction between hydroxylated alkyd resins and urea formaldehyde resin, either by super acidic catalyst with H+ protons or in furnace conditions, always water and amounts of methanol and normal Butanol is released as a side product in the form of an exit gas from the reaction. It is for this reason that in general, acid cure polyesters or acid cures face the problem of drying and hardening in humid atmospheric conditions, because the humidity of the environment prevents the progress of the reaction due to the release of water, but polyurethane is not like this because the mechanism Polyurethane reaction does not have a specific output, and on the other hand, the stability of the urethane bond energy level, which includes amide-ester bond pairs, is very high, and this is the factor that causes their chemical resistance.
In polyurethane, we are dealing with two parts, one part A which contains hydroxyl resin and the other part B which contains isocyanate resin. To fully understand polyurethane, it is better to know each component well:
Types of component A: Any resin that has excess OH excess hydroxyl group in such a way that at least 30% of the hydroxyl value of that resin including type 1 hydroxyl is available can participate in the polyurethane formation reaction.
Therefore, three types of resins, including acrylic polyol, alkyd polyol, and polyester polyol, will be the most widely used component A resins in the formation of polyurethane. Each of these resins will have specific applications and advantages in polyurethanes, which include the following:
Polyol Alkyd: Short oil alkyds (soybean and castor oil) are hydroxyl-containing, which are mainly used in the production of polyurethane paints for wood industries, because alkyds are oil-based and face the problem of yellowness, and are mostly used in indoor spaces that they are not exposed to direct light. But their advantage is its compatibility and stickiness to cellulose fibers of wood, on the other hand, polyurethane derived from alkyd polyols are quick drying and anti-moisture and give a kind of classic beauty to wooden artifacts.
Polyester polyol: Polyester is the alkyd resin that has synthetic acids instead of fatty acid. The light resistance and chemical resistance of polyester is much higher than alkyds. Urethane must have hydroxyl type 1 available because polyurethanes derived from polyester polyols are a little slow drying and therefore are mainly used with aromatic isocyanate hardeners and for indoor use free of direct light or for the production of polyurethane primers or polyurethane floors.
Acrylic polyol: are resins with a linear and flexible structure and with the least amount of aromatic monomers, which are produced through a radical mechanism from several unsaturated acrylate monomers, monostyrene and hydroxylated acrylate monomers. All hydroxyl groups are available in acrylic resin and they all participate in polyurethane reaction. An important feature of acrylic polyol is its optical and chemical resistance, and it is mainly used in the production of polyurethane topcoats and varnishes in automotive paints, as well as the final topcoat color in the oil and petrochemical industries. And of course, sometimes with modifications, it will also be able to be used in woodworking and decoration.



Types of component B: Polyisocyanate hardeners include oligomers and trimers and dimers of several monomers with isocyanate groups and include the following three types.
Aromatic: maleic diisocyanate TDI, MDI toluene diisocyanate
Aliphatic: HDI hexadiisocyanate, HDMI hexadimethylisocyanate
Cycloaliphatic: Isofuran diisocyanate IPDI
Each spectrum of polyisocyanates is produced and supplied by a specific type of mechanism called: adduct, biuret, trimer.
Whenever we are looking for hardness and fast drying properties in polyurethane paint, we should use aromatic adduct isocyanate hardener, of course, due to aromaticity in TDI and the resonance of the electron pair of nitrogen N in its structure, the light resistance is reduced and causes yellowing. This problem is a thermodynamic behavior. And cannot be fixed with anti-UV and HALS additives.
If our goal is high flexibility and light resistance along with high atmospheric resistance, then aliphatic and cycloaliphatic hardeners should be used, only these types of polyurethanes dry and harden more slowly, but they reach their best quality after 210 hours.
In any case, in polyurethane, mainly polyester polyol and alkyd polyol are hardened with TDI and MDI hardeners, and acrylic polyols are hardened with HDI, HMDI, and IPDI aliphatic and cycloaliphatic hardeners.
Note that alkyd and polyester due to having type 3 hydroxyl caused by glycerol monomer in them practically due to steric hindrances, part of hydroxyl never reacts with aliphatic isocyanates, therefore the best option for them is to use aromatic and active isocyanates.
Now that we know the mechanism of polyurethane, we know that what prevents the hardening of a polyurethane coating will be a factor called harmful hydroxyl in the environment.
In this article, we have described polyurethanes in detail, and today, Rangin Kimia Company is proud to be the first Iranian producer of polyurethane paints specialized in the wood industry, according to customer needs, in improving the quality of the final work, it has researched and developed for years and the list of products It supplies 30 types of polyurethane coatings to Iran and the Middle East market.

Production and collection: Rangin Kimia Industrial Complex
All rights of this article are reserved for Rangin Kimia.